Abstract
Background:
Patients with PNH have uncontrolled terminal complement activation that can lead to thrombosis, organ damage, intravascular hemolysis (IVH), and clinical sequelae. It is also associated with debilitating patient-reported outcomes (PROs), such as fatigue, dyspnea, and pain that contribute to a poor quality of life (QoL). Whilst it is known that improvements in clinical outcomes are associated with C5 inhibitor (C5i) therapy in patients with PNH, evidence characterizing the relationship between clinical outcomes and fatigue or QoL are limited. Understanding key clinical drivers of improvements in QoL and fatigue during complement C5i therapy is vital for developing appropriate management strategies.
Aims:
To assess the relationship between clinical outcomes with fatigue and QoL, as measured by Functional Assessment of Chronic Illness Therapy - Fatigue (FACIT-F) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire - Core 30 Global Health (EORTC QLQ-C30 GH), in patients with PNH receiving C5i therapy.
Methods:
Post-hoc analyses were performed using data from a 26-week data cut of a randomized phase 3 study (NCT02946463) that assessed ravulizumab and eculizumab in complement inhibitor-naïve patients with PNH and high disease activity (defined as a lactate dehydrogenase [LDH] level ≥ 1.5 × upper limit of normal [ULN; 246 U/L] and ≥ 1 sign or symptom of PNH at screening). The PRO measures (PROMs) used were FACIT-F and EORTC QLQ-C30 GH. Clinical variables included LDH, hemoglobin (Hb), bone marrow disorders, transfusions, and hematological parameters such as reticulocyte, platelet, and neutrophil counts. Multivariable regressions were performed separately for each PROM to assess the effect of clinical variables on PROM score changes from baseline to day 183, controlling for demographic characteristics and baseline PROM scores. Multicollinearity between covariates was tested in each regression model and removed when present.
Results:
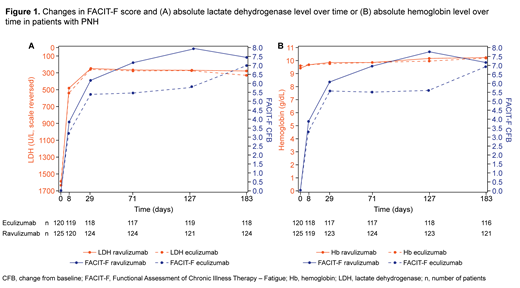
Data for 121 and 125 patients with PNH treated with eculizumab or ravulizumab were included, respectively. Trial data showed that reduced LDH levels at day 183 were associated with improvements in FACIT-F in both treatment groups; however, no equivalent association was observed with Hb levels (Figure 1). In the regression analyses, significant predictors of FACIT-F improvement included reductions in LDH levels from baseline to day 183 (p = 0.0024) and the interaction of both achieving a LDH level ≤ 1.5 × ULN by day 183 and improvements in Hb from baseline (p = 0.0285). Similarly, significant predictors of EORTC QLQ-C30 GH improvement also included reductions in LDH levels from baseline to day 183 (p < 0.0001) and an increase in Hb from baseline to day 183 after receiving a transfusion during the study period (p = 0.02). However, Hb as a main effect, whether as an improvement in Hb levels from baseline to day 183, or Hb values at baseline, were not statistically significant predictors of improvement in either PROM at day 183.
Conclusions:
In this analysis, key clinical drivers of improvement in PROMs were determined among patients with PNH receiving C5i therapy. When multiple clinical variables were considered, reductions in LDH were one of the strongest predictors of improvements in fatigue and QoL. Increases in Hb levels from baseline were only a significant predictor of improvement in FACIT-F for patients who had attained LDH level ≤ 1.5 × ULN at day 183, highlighting the importance of controlling IVH in patients with PNH. Finally, these results suggest that Hb alone is not a strong predictor of improvements of fatigue and QoL in this disease setting.
Schrezenmeier: Alexion, AstraZeneca Rare Disease: Honoraria, Other: Travel support, Research Funding; Apellis: Honoraria; Roche: Honoraria; Sanofi: Honoraria; Novartis: Honoraria. Kulasekararaj: F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Speakers Bureau; Apellis: Consultancy; Akari: Consultancy, Honoraria, Speakers Bureau; Biocryst: Consultancy, Honoraria, Speakers Bureau; Achilleon: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria, Speakers Bureau; Ra Pharma: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion, AstraZeneca Rare Disease Inc.: Consultancy, Honoraria, Other: Travel support. Mitchell: Alexion, AstraZeneca Rare Disease Inc.: Honoraria. Peffault De Latour: Jazz Pharmaceuticals: Honoraria; Amgen: Consultancy, Other, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding. Devos: AbbVie: Consultancy; Alexion, AstraZeneca Rare Disease Inc.: Consultancy; Incyte: Consultancy; Novartis: Consultancy; Bristol Myers Squibb - Celegene: Consultancy. Okamoto: Alexion, AstraZeneca Rare Disease Inc.: Honoraria, Research Funding. Wells: Alexion, AstraZeneca Rare Disease Inc.: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Johnston: Broadstreet HEOR: Current Employment. Popoff: Broadstreet HEOR: Current Employment. Cheung: Broadstreet HEOR: Current Employment. Wang: Alexion, AstraZeneca Rare Disease Inc.: Current Employment. Gustovic: Alexion, AstraZeneca Rare Disease: Current Employment. Wang: Alexion, AstraZeneca Rare Disease: Current Employment. Tomazos: Alexion, AstraZeneca Rare Disease: Current Employment. Patel: Alexion, AstraZeneca Rare Disease Inc.: Current Employment. Lee: Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal